blog
HOYA (TOKYO:7741) today announced that it has signed an agreement to acquire a majority shareholding in Wassenburg Group, an Automated Endoscope Reprocessing (AER) market leader in the Netherlands. The planned…
Hoya Corporation is a Japanese company manufacturing optical products such as photomasks, photomask blanks and glass magnetic-memory disks, contact lenses and eyeglass lenses using wavefront technology for the health-care market,…
PENTAX Medical Singapore Pte. Ltd. signed Distribution Agreement with Wassenburg Medical Devices for Asia Pacific Region Shanghai, April 16th, 2013 – PENTAX Medical Singapore Pte. Ltd., a wholly-owned subsidiary of…
HOYA will acquire the majority stake in the automated endoscope reprocessing company Wassenburg Group, based in the Netherlands, according to an A to Z Optics/Photonics report. The PENTAX Medical division…
Already 1896, PARTSCH described the use of apicoectomy in the surgical treatment of chronic apical periodontitis. Amputation of the apical third of the root with its ramifications and excochleation of…
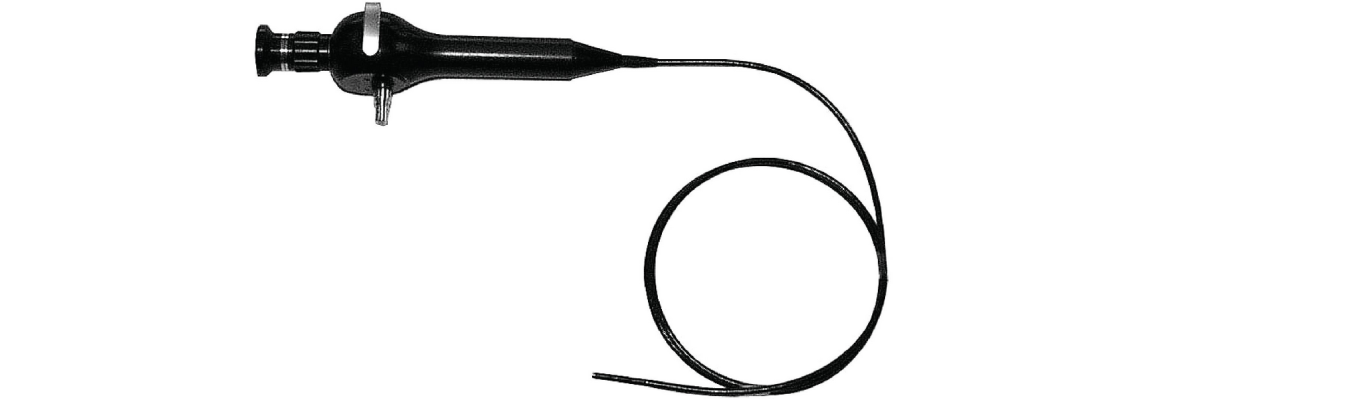
Borescopes can be rigid or semirigid. A rigid borescope, also known as rigid endoscope or swing-prism endoscope, is an endoscope that uses a lens system for image transmission. A semirigid…
For KARL STORZ this year’s MEDICA – the largest international trade fair for medical technology – started with important guests. On November 17, 2010, the opening day, Dr. Sybill Storz…
Leading supplier in endoscopy and OR integration KARL STORZ has developed the first true uncompressed digital operating room platform via IP. This networked approach allows complete integration of all video…
On November 11, 2010, Minister-President Stefan Mappus presented Dr. Sybill Storz with the Federal Cross of Merit, which is awarded by the former German President Horst Köhler. During the ceremony…
CULVER CITY, CA (July 16, 2008) – KARL STORZ Endoscopy-America, Inc., a global leader in minimally invasive endoscopic technologies and operating room integration, has announced an exclusive OR integration distribution…